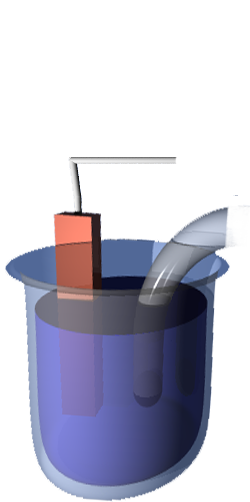
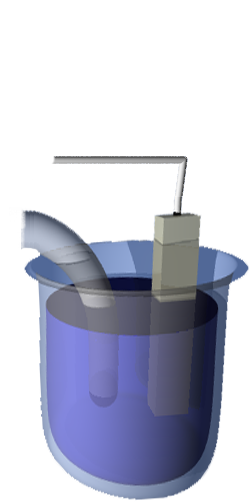
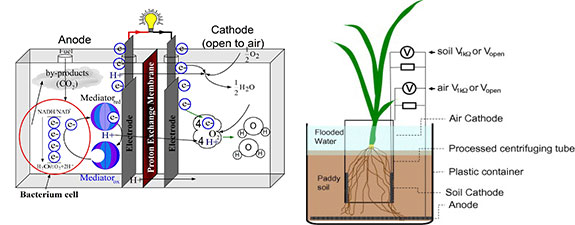
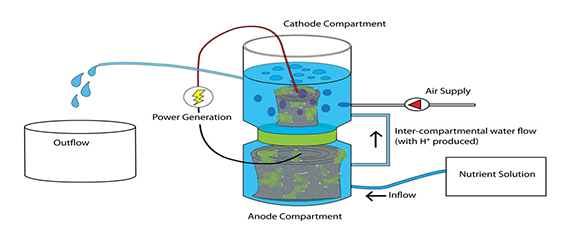
Ideal Performance
The ideal performance of an MFC depends on the electrochemical reactions that occur between the organic substrate at a low potential such as glucose and the final electron acceptor with a high potential, such as oxygen. However, its ideal cell voltage is uncertain because the electrons are transferred to the anode from the organic substrate through a complex respiratory chain that varies from microbe to microbe and even for the same microbe when growthconditions differ. Though the respiratory chain is still poorly understood, the key anodic reaction that determines the voltage is between the reduced redox potential of the mediator (if one is employed) or the final cytochrome in the system for the electrophile/anodo-phile if this has conducting pili, and the anode. For those bacterial species that are incapable of releasing electrons to the anode directly, a redox mediator is needed to transfer the electrons directly to the anode. In such a case the final anodic reaction is that the anode gains the electrons from the reduced mediator.
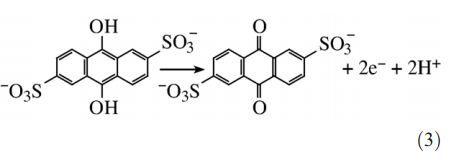 Eq. illustrating Anodic
Eq. illustrating Anodic
Reaction with AQDS
The Equation in Figure illustrates the anodic reaction with AQDS (on the right side of the equation), the major component of the humics, as the mediator. The anodic potential is consequently defined by the ratio of AHQDS and AQDS.
In mediator-less MFCs utilizing anodophiles such as G. sulfurreducensand and R. ferrireducens microbes form a biofilm on the anode surface and use the anode as their end terminal electron acceptor in their anaerobic respiration. Section 2 mentioned the possible electron transport process. Though the respiratory chain is still not well understood, the anodic potential can be evaluated by the ratio of the final cytochrome of the chain in reduced and oxidized states. The electrode reactions for various types of MFCs and their cor-responding redox potentials of those substrates involved in electrode reactions are presented in Table. The ideal potentialsof MFCs can be calculated by the Nernst equation forthese reactions and they range from several hundred mV to over 1000 mV.
Actual Performance
The actual cell potential is always lower than its equilibrium potential because of irreversible losses. The following equation reflects various irreversible losses in an actual MFC.
Vcell = Ecathode - |nact,c + hconc,c| - EAnode - |nact,a + hconc,a| - iRi
where nact,a + hconc,a are activation polarization losses on cathode and anode, respectively. nact,c + hconc,c are concentration polarization in cathodic and anodic chambers, respectively. Ohmic losses nohm occur because of resistance to the flow of ions in the electrolyte and resistance to flow of electrons through the electrode. Since both the electrolyte and the electrodes obey Ohm's law, it can be expressed as iRi,in which i is the current flowing through the MFC, and Ri is the total cell internal resistance of the MFC.
Activation polarization is attributed to an activation energy that must be overcome by the reacting species. It is a limiting step when the rate of an electrochemical reaction at an electrode surface is controlled by slow reaction kinetics. Processes involving adsorption of reactant species, transfer of electrons across the double-layer cell membrane, desorption of product species, and the physical nature of the electrode surface all contribute to the activation polarization. For those microbes that do not readily release electrons to the anode, activation polarization is an energy barrier that can be overcome by adding mediators. In mediator-less MFCs, activation polarization is lowered due to conducting pili. Cathodic reaction also faces activation polarization. For example, platinum (Pt) is preferred over a graphite cathode for performance purpose because it has a lower energy barrier in the cathodic oxygen reaction that produces water. Usually activation polarization is dominant at a low current density. The electronic barriers at the anode and the cathode must be overcome before current and ions can flow.
The resistance to the flow of ions in electrolytes and the electron flow between the electrodes cause Ohmic losses. Ohmic loss in electrolytes is dominant and it can be reduced by shortening the distance between the two electrodes and by increasing the ionic conductivity of the electrolytes. PEMs produce a transmembrane potential difference that also constitutes a major resistance.
Concentration polarization is a loss of potential due to the inability to maintain the initial substrate concentration in the bulk fluid. Slow mass transfer rates for reactants and products are often to blame. Cathodic overpotential caused by a lack of DO for the cathodic reaction still limits the power density output of some MFCs. A good MFC bioreactor should minimize concentration polarization by enhanc-ing mass transfer. Stirring and/or bubbling can reduce the concentration gradient in anMFC. However, stirring and bubbling requires pumps and their energy require-ments are usually greater than the outputs from the MFC. Therefore, balance between the power output and the energy consumption by MFC operation should be carefully considered. A polarization curve analysis of an MFC can indicate to what extent the various losses listed in above contribute to the overall potential drop. This can point to possible measures to minimize them in order to approach the ideal potential. These measures may include selection of microbes andmodifications toMFC configurations such as improvement in electrode structures, better electro-catalysts, more conductive electrolyte, and short spacing between electrodes. For a given MFC system, it is also possible to improve the cell performance by adjusting operating conditions.